Abstract
Introduction T-cell prolymphocytic leukemia (T-PLL) is an aggressive and uncommon T-cell malignancy, characterized by treatment refractoriness, early relapse, and poor prognosis. Limited data are available characterizing T-PLL patients (pts) and outcomes in the modern era. We aimed to evaluate national trends in survival outcomes with the advent of novel therapies and features associated with disparate treatment access and survival.
Methods T- PLL pts diagnosed from 2004-2019 were identified in the National Cancer Database. Demographic and first line treatment (limited to treated, active surveillance, or not treated) characteristics were identified, with logistic regression for odds of receiving treatment at time of diagnosis, referenced to pts who remained untreated due to early death, palliative care, or contraindication. The utilization of immunotherapy was identified for pts diagnosed after 2012, corresponding to the recoding of alemtuzumab as immunotherapy. Kaplan-Meier and Cox regression analysis were used to compare overall survival (OS) by year (yr) of diagnosis, with diagnosis in 2004-2006 considered to be prior to the availability of alemtuzumab and diagnosis 2017 or later considered as having access to venetoclax.
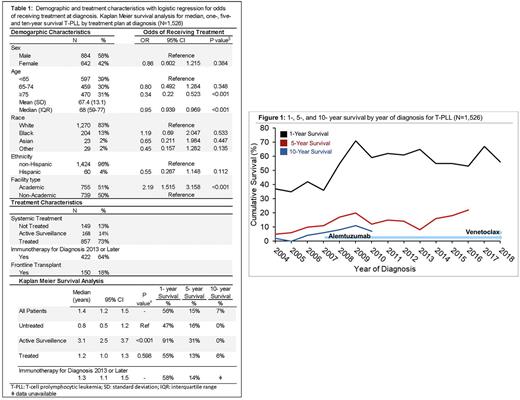
Results Of the 1,526 T-PLL pts identified, the median age at diagnosis was 68 yrs (interquartile range 59-77). T-PLL pts were more likely to be male (58%) and White (83%). Pts did not receive treatment in 13% of cases (N=149), with 14% managed with active surveillance (N=168). For treated pts, 18% of T-PLL cases were treated with frontline transplant, and for pts diagnosed 2013 or later, 64% (N=422) of T-PLL pts received immunotherapy front line. T-PLL pts were more likely to receive treatment if they were managed at academic vs non-academic centers (OR 2.19 [95% confidence interval (CI) 1.52-3.16]; p<0.001), and less likely to receive treatment with increased age (OR 0.95 [95% CI 0.94-0.96]; p<0.001), Charlson-Deyo score of 2 or greater (OR 0.48 [95% CI 0.30-0.77]; p=0.002), or if uninsured (OR 0.22 [95% CI 0.09-0.59]; p=0.002).
With a median follow up of 1.1 yrs, T-PLL pts had a median OS of 1.4 yrs (95% CI 1.2-1.5), with a 1-, 5-, and 10-yr survival of 56%, 15%, and 7%, respectively. Pts managed with active surveillance had a median OS of 3.1 yrs (95% CI 2.5-3.7 yrs, p<0.001 referenced to untreated pts). T-PLL pts offered upfront treatment had a median OS of 1.2 yrs (95% CI 1.0-1.3, p=0.598), with a 1-,5-, and 10- yr OS of 55%, 13%, and 6%, respectively. For the 28% of pts who were diagnosed after 2012 and treated with immunotherapy, median OS was 1.3 yrs (95% CI 1.1-1.3). On multivariable Cox regression, academic center management was associated with improved OS (hazard ratio (HR) 0.86 [95% CI 0.76-0.97], p=0.013), and factors associated with reduced OS included increasing age (HR 1.02 [95% CI 1.01-1.03], p<0.001) and increasing comorbidity score (HR 1.20 [95% CI 1.11-1.30], p<0.001).
When examining survival trends by year of diagnosis, patients diagnosed 2007 or later had improved OS over the pre-alemtuzumab period, with median OS of 1.4 years (95% CI 1.2-1.5) for diagnosis from 2007-2016 vs 0.7 yrs (95% CI 0.5-0.9 years) from 2004-2006 (p<0.001). The venetoclax period, from 2017-2019, had a median OS of 1.5 years (95% CI 1.2-1.7, p<0.001, referenced to diagnosis from 2004-2006).
Conclusion We present the largest study to date on T-PLL, identifying current real-world survival outcomes. While there have been small improvements over time, which may correlate with the introduction of alemtuzumab and venetoclax, survival remains dismal. A small subset of pts managed with active surveillance had improved survival, though this likely represents pts with asymptomatic "inactive T-PLL", who can remain untreated until progression. While limited by the retrospective nature of this study, we found no significant survival benefit for pts who received treatment compared to those who received no treatment due to palliative care, comorbidity, or early death. This suggests that all pts should be offered options of clinical trials and palliative care at diagnosis. Due to the significant survival advantage observed for patients managed at academic centers, prompt and early referrals to experienced clinicians should be considered. Given the aggressive nature of this disease, persistent short OS despite novel therapies, innovative treatment options are paramount to improve outcomes.
Disclosures
Shah:AbbVie: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Epizyme: Research Funding; ADCT: Research Funding; BeiGene: Research Funding; Astrazeneca: Research Funding. Hu:Bristol Meyers Squibb: Consultancy; Novartis: Consultancy; ADC Therapeutics: Consultancy; Lymphoma Research Foundation: Research Funding; Genentech: Research Funding; Celgene: Research Funding; CRISPR Therapeutics: Research Funding; Caribou Biosciences: Research Funding; Morphosys AG: Research Funding; Repare Therapeutics: Research Funding. Stephens:Celgene: Consultancy; Genentech: Consultancy; Lilly: Consultancy; TG Therapeutics: Consultancy; Epizyme: Consultancy; Newave: Research Funding; Beigene: Consultancy; CSL Behring: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Novartis: Research Funding; Mingsight: Research Funding; Karyopharm: Research Funding; JUNO: Research Funding; Arqule: Research Funding; Acerta: Research Funding.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal